
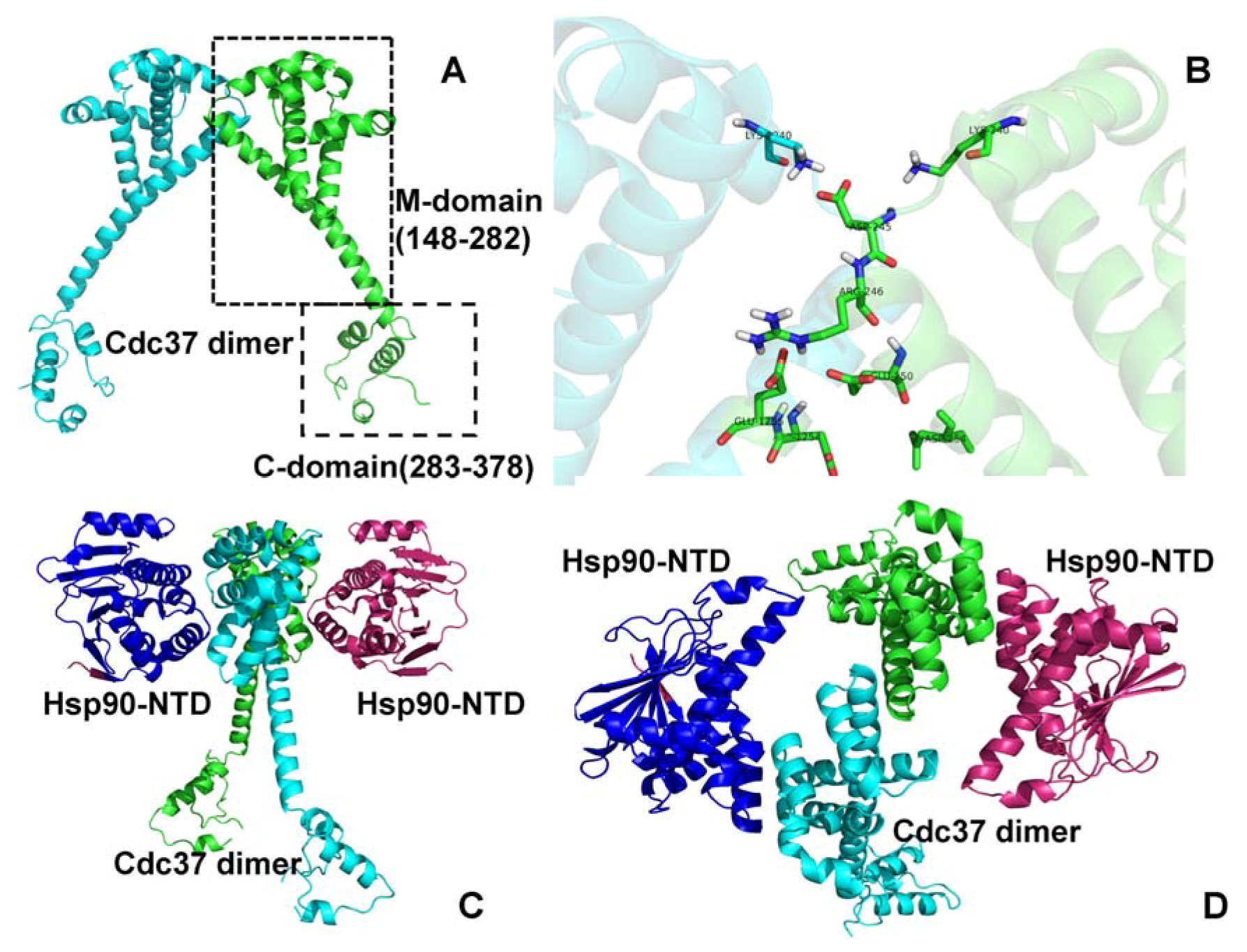
One difference is in the conformation: HSP90 of the tumor phenotype is more susceptible to inhibitors. Differences between normal HSP90 and HSP90 of the tumor phenotype have been better understood and have aided in making the chaperone protein a target for cancer drugs. Despite this observation, the possible role of HSP90 in cancer was overlooked because the chaperone was also present in extreme amounts in normal cells and was vital to normal cell function, as observed when the drastic adverse effects resulting from gene knockout inhibited the production of this protein. Elevated levels of HSP90 have been observed in patients with cancer. Various co-chaperones are necessary for HSP90 to function. HSP90 is also secreted into an extracellular environment via an exosome pathway that differs from the classic secretion pathway. Isoforms HSP90 α and HSP90 β are present in the cytoplasm TRAP1 is present in the mitochondria and GRP94 is present in the endoplasmic reticulum and is likely secreted due to post-translational modifications (PTM). These isoforms are present both within the cell and outside the cell. Multiple isoforms of HSP90 exist, and these isoforms share high homology. Structurally, this protein is a dimer with monomer subunits that consist of three main conserved domains known as the N-terminal domain, middle domain, and the C-terminal domain. As a chaperone protein, it correctly folds client proteins. HSP90 is a vital chaperone protein conserved across all organisms.


 0 kommentar(er)
0 kommentar(er)
